- Fourth, challenges to evidence-based research into the effectiveness of
personalized medicine include the need for large cohorts and longitudinal data
collection to generate sufficient data in order to compute the treatment effect and
gauge the potential costs and benefits.
- Fifth, there are also consent and privacy issues that come into play in large cohort
studies. In addition, genetic studies of large cohorts require significant biobanking,
genotyping and information technology infrastructure.
Personalized Medicine Depends on Biospecimen (tissue) Donation
Personalized medicine for cancer is in many cases still a vision for the future. For it to
become a greater reality, cancerous and normal biospecimen samples will need to be:
- Donated by willing, informed individuals
- Collected, processed and stored in a standardized format
- Tested by both conventional pathology methods and genomic profiling
- Evaluated in the context of a patient's clinical medical history and their
environmental exposure history.
Based on this information, a patient will be treated with therapies or approaches that
target the cancer preferentially, while sparing normal tissue.
For more information about
the importance of tissue donation from CISN go to:
http://cisncancer.org/research/how_cancer_is_studied/translational/biospecimen_issues.html
Standardization of Biospecimens is Crucial to Success
1. Patient Consent
Health professionals explain to the patient that their tissue donation is very important to
accelerating progress in cancer research. If / when permission is given, the consent form
is archived carefully and uniquely linked to the sample.
Advocates can play a role in this process by educating the public about the importance of
tissue donation. This is an individual's choice and although the progress of personalized
medicine depends on tissue collection, no pressure should be brought by either advocates
and/or doctors to influence a person in their decision.
The consent document needs to be understandable to the person being approached with
implications for future use and confidentiality/privacy clearly explained.
2. Sample collection
It is critically important to understand how to collect cancer biospecimens properly, so
that the information obtained from analyses, using approaches such as genome-wide
profiling, are correct and not artifacts of mishandling.
3. Obtain and Stabilize Sample
A biospecimen is removed from the patient, taking great care to keep the sample
biologically viable. These steps need to be standardized across the Country so in
research, samples can be compared against each other to determine normal values and in
the clinic, so values reported out to patients are valid and can be reproduced wherever
that patient goes for treatment.
4. Attach unique identifier
A unique identifier is associated with the biospecimen. Unique identifiers link the tissue
sample with its relevant annotated records, so future results from studies using this
sample can again be associated with the original patient donor. Confidentiality must be
adhered to.
5. Store carefully
A biospecimen is carefully stored in a location that monitors temperature and conditions
to properly support the integrity of the tissue.
6. Retrieve and re-store carefully
When needed for analysis, a biospecimen is carefully retrieved, and if any sample remains
afterward, it is re-stored with care.
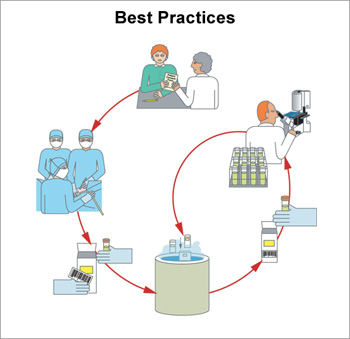
7. Best Practices
Examples of Large Personalized Medicine Programs
We have listed several large-scale personalized medicine programs below to demonstrate
work in progress. As you read through them you learn that there is quite a variety of
approaches, some more broad than others.
1. The Coreill Personalized Medicine Collaborative CPMCT is enrolling individuals in a
research study to investigate the impact of personalized medicine on health outcomes.
| |
|
 |
Over the next several years, study participants will receive
personalized genetic results for their risks of complex diseases
such as cancer, heart disease and diabetes.
Participants will be asked to complete follow-up questionnaires
to inform the medical and scientific community about the utility
of using genome information in medical care.
The CPMC is an evidence-based research study designed to
determine which elements of personal genetic data are valuable
in clinical decision-making and healthcare outcomes. |
| Image courtesy of CPMC: http://cpmc.coriell.org/ |
|
As of 2011, the CPMC are enrolling participants into wellness and cancer
arms. Close partnerships with area hospitals are designed to catalyze physician
engagement in personalized medicine.
2. The Personalized Medicine Coalition published the second edition of 'The Case for
Personalized Medicine' in May 2009. This report details how personalized medicine plays
an increasingly integral role in delivering high-quality, cost-effective healthcare and
presents evidence that personalized medicine will continue to grow in importance as
scientific breakthroughs are translated into a new generation of targeted therapeutics.
For more about this coalition go to: http://www.ageofpersonalizedmedicine.org/index.asp
3. Wisconsin Genomics Initiative (WGI)
On October 10, 2008, Wisconsin Governor Jim Doyle announced the Wisconsin Genomics
Initiative, which is a collaborative research effort among Marshfield Clinic, Medical College
of Wisconsin, University of Wisconsin School of Medicine and Public Health, and University
of Wisconsin-Milwaukee.
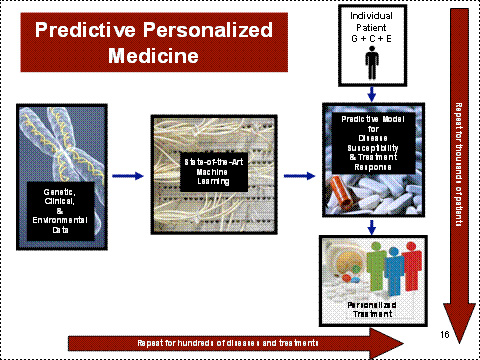
The vision of WGI is to be able to predict for individual patients in a clinical setting the
risks of disease susceptibility and treatment response using the combined power of cutting-edge genetic, phenotypic, and environmental analyses, thereby making the
promise of personalized medicine a reality. (figure below)
The key elements of the WGI strategy are to:
1) Genotype up to 20,000 participants for 1,000,000 genetic markers
2) Validate selected target phenotypes and multiple clinical attributes from the Marshfield
Clinic.
3) Integrate genetic, phenotypic, and environmental information databases and develop
the search engines to use data efficiently for scientific discovery
4) Build predictive computational models using machine learning and super-computer
capability, for the key equation, Genetic + (Environment and Clinical) = Phenotype.
5) WGI will then conduct initial predictive studies (diabetes, obesity, coronary artery
disease, and atrial fibrillation) to test and improve the scientific platform, as well as a
genome-wide association study (GWAS). For more information go to:
http://www.hhs.gov/myhealthcare/news/community.html
4. Partnership for Personalized Medicine (PPM)
PPM is a nonprofit initiative whose goal is the development, validation and clinical
application of new molecular diagnostics designed to improve health outcomes and,
importantly, reduce health care costs.
The Partnership for Personalized Medicine is led by Dr. Lee Hartwell, President and
Director of the Fred Hutchinson Cancer Research Center and 2001 Nobel laureate; Dr.
Jeffrey Trent, President and Scientific Director of the Translational Genomics Research
Institute (TGen); and Dr. George Poste, Director of the Biodesign Institute at Arizona State
University.