The Promise of Personalized Medicine
How will obtaining my genetic blueprint benefit me? Why find that I am more susceptible
to getting a disease that has no cure? These are relevant questions that are now being
asked by the public, as researchers continue to unfold the mystery of the human genome.
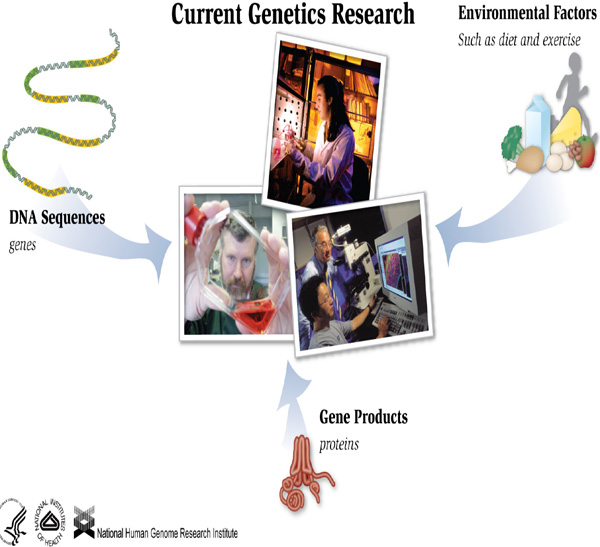
Researchers have found that cancer reflects the interplay between an individual's
environment and their genes. However they are still in the initial stages of understanding
the specific role each plays. Thus, in the near future, public health genomics, and more
specifically environmental health, will become an important part of the future healthcare-related
issues.
To date, much of the promise and pitfalls of personalized medicine remain untested. The
study of genetic variation has proven to be much more complex than ever imagined.
Proteomics and metabolomics are still in the early stages of study and not many
examples are in use today. Yet, advocates of personalized medicine have stressed its
potential to:
| |
|
 Image provided by CISN archives. All rights reserved. Image provided by CISN archives. All rights reserved. |
- Select optimal therapy and reduce
"trial-and-
error"
- Reduce adverse drug reactions
- Improve the selection of drug targets
- Reduce the time, cost, and failure rate of
clinical trials
- Revive drugs that failed clinical trials in
large groups and retest in correct subgroup
- Find reliable tumor markers that detect
disease early
- Shift the emphasis in medicine from reaction
to prevention and reduce the overall cost of
healthcare.
|
| |
|
Examples of Personalized Medicine for individuals
Below are just a few of the examples in use today. This list is not inclusive and changes
quickly as the science moves forward.
1. Testing for Disease-Causing Genetic Mutations
- The BRCA1 and BRCA2 genes are implicated in familial breast and ovarian cancer
syndromes. Discovery of a disease-causing mutation in a family can inform "at-risk" individuals as to whether they are really at higher risk for cancer and may prompt
individualized prophylactic therapy.
- The discovery of a chromosomal translocation in chronic myelogenous leukemia led
to the development of a drug (imatinib) that targets the enzyme produced as a
result of that mutation.
2. Targeted Therapy is the use of medications designed to target aberrant molecular
pathways in a subset of patients with a given cancer type. For example,
- Herceptin is used in the treatment of women with breast cancer in which HER2 is
over expressed.
- Gleevec is a Tyrosine kinase inhibitors developed to treat chronic myeloid leukemia
(CML), in which the BCR-ABL fusion gene (the product of a reciprocal translocation
between chromosome 9 and chromosome 22) is present in >95% of cases.
These medications are a prime example of "rational drug design" based on knowledge
of disease pathophysiology.