What do advocates do in CLINICAL RESEARCH?
Clinical research involves patients, so the inclusion of advocates and patients is very
important. Most researchers, institutions and funders agree on many aspects of
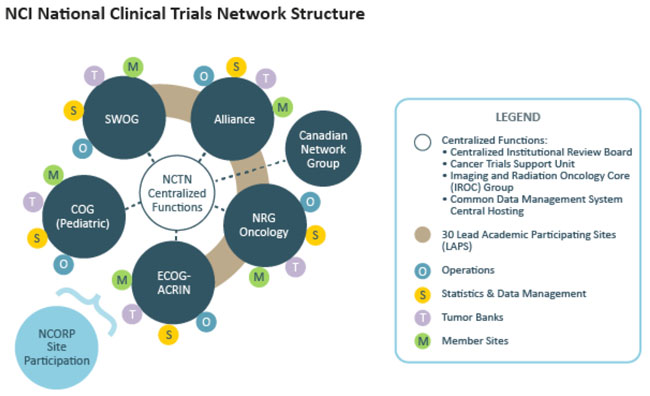
advocate involvement in clinical research. The government run National Clinical
Trials Network is one area in which advocate involvement is readily seen.
Patient advocates are involved in all aspects of clinical trial development through the
National Clinical Trials Network (supported by grants from the National Cancer
Institute; see information box) to provide the patient’s perspective throughout the
development and implementation of clinical trials by:
- Initiating strategies that accelerate study development, activation, accrual
participation and reporting results
- Aiding in the selection of cancer research concepts to study
- Fostering collaboration and interactions between and among committees
- Ensuring that clinical trials are both patient centered (lead to improved clinical
outcomes) and respect patients
Patient advocates can also be involved locally at Institutions in their area (e.g. NCI
designated Comprehensive Cancer Centers, Community Cancer Centers). Locally there are many ways that advocates can help to make sure trials accrue, there are no
barriers to patient participation and that patients are fully informed before they
consent to be on a clinical trial.
Below are a few activities of advocates in clinical research at local cancer centers.
- Every Comprehensive Cancer Center should have a Protocol Committee that
reviews every study before it is sent to the Institutional Review Board for final
approval.
 |
|
The advocate role on this committee can vary, but may include ensuring readability and accuracy of
the consent form and the identification of potential
patient barriers to accrual. |
|
- Every institution that conducts research in people (clinical trials) has an
Institutional Review Board (IRB). This board reviews every clinical trial to
assure that appropriate steps are taken to protect the rights and welfare of
people participating in clinical trials (FDA web-site). There is always a
consumer advocate on the IRB at any institution conducting clinical trials.
- Patients can be members of local Data Safety Monitoring Boards (DSMB)
- DSMBs are assembled for most clinical trials to provide oversight and
monitoring of the conduct of a clinical trial to ensure the safety of participants
while maintaining integrity of the data. They are an independent group that
can look at the data for undue toxicity or futility (an arm is clearly inferior),
without compromising the data. Advocates can participate as voting members
of these boards.
- Advocates can become involved with their health care providers in many ways.
One way advocates become involved is through navigation of other patients
through the system. Many advocates also facilitate support groups and
maintain a high level of provider and patient interactions.
Why is it important?
It is very important to include advocates in clinical research projects to ensure the
patient point of view is heard during the conduct of this type of research.
 |
|
Advocates also understand the shock and fear patients
experience after a cancer diagnosis and how difficult it is to
make treatment decisions. They can help to develop strategies to assist a patients
understanding of clinical trials. |
|
Additional important contributions are listed below:
- Inclusion of advocates in clinical research decisions fosters public trust.
- Advocates can see barriers to accrual through a patient’s viewpoint and
ensure that they are removed or minimized.
- Advocates help to communicate information about clinical trials by:
- Engaging the broader advocacy community and public
- Developing online resources to educate the public on the availability
of clinical trials as an investigational (still be studied) treatment
option
- Developing patient centered materials and encouraging physicians to
discuss trials with patients
- Publishing and disseminating clinical trial results in plain language to
the general public
Advocates in Drug Development
The Food and Drug Administration (FDA) is an agency within the US Department of
Health and Human Services and has many responsibilities including protecting the
public health by assuring the safety, effectiveness, quality and security of human
and animal drugs, vaccines, biological products and medical devices.
Part of the FDA’s mission is to evaluate new therapies and determine which are safe
and effective for their intended use. This is a complex job, often involving many
areas of expertise, and sometimes the FDA turns to outside experts for counsel.
FDA’s advisory committees provide independent, expert advice to the agency on a
range of complex scientific, technical, and policy issues.
What do advocates do in the FDA?
The FDA has involved advocates in many different ways. The FDA has many
different types of meetings and involves patients and consumers during these
meetings.
- Some of the meetings are early in drug development and include meetings
with the sponsor about how best to move a drug into the approval process.
- Some meetings are during the drug approval process to monitor ongoings
- Others are to evaluate different ways of approving drugs or changes to the
approval process.
To include research advocates the FDA recruits Patient Representatives (who are
Special Government Employees) to participate as consumer and patient advocates on
many different kinds of FDA committees.
Why is it important?
One very important role for advocates at the FDA is when the data is submitted for
the approval of an agent but the results are not clearly positive or negative. At this
point they may initiate an Oncologic Drugs Advisory Committee (ODAC) meeting.
This committee includes top scientists in the field as well as a consumer
representative and a patient representative. Advocates who are survivors can
participate as either. Those who are not survivors would only be able to be a
consumer representative. The perspective of the patient and consumer is not to be
contradictory, but is to evaluate the evidence and ensure the correct decision is
made on behalf of all patients that may benefit from the new agent.
It is extremely important for the patient and consumer representatives to be well
informed and have the ability to understand the disease being reviewed and how it
is currently treated.
Below are some benefits patients and consumers bring to an FDA meeting:
- Representatives for industry, patients, and consumers add different
perspectives and expertise that give balance to the discussions and final
recommendations.
- Patient and consumer representatives facilitate dialogs that affect consumers.
- Patients can evaluate the safety and effectiveness of products that are under
review, so they need an ability to understand research and scientific data.
- Evaluating risk versus benefit from a patient perspective is important (see
section on risk/benefit).
|