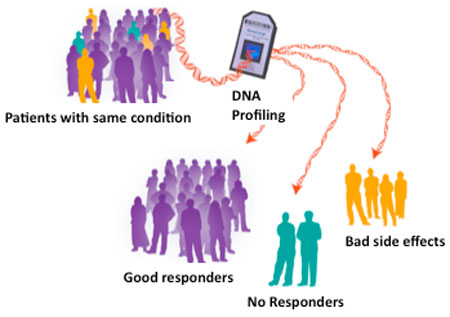
The wider use of pharmacogenomic testing is viewed by many as an outstanding
opportunity to improve drug prescribing safety and efficacy.
Driving this trend are the 106,000 deaths and 2.2 million serious events caused by
adverse drug reactions in the US each year (Lazarou 1998).
What Pharmacogenomics Means For People?
Until recently, drugs have been developed with the idea that each drug works pretty
much the same in everybody. But genomic research has changed that 'one size fits all'
approach and opened the door to more personalized approaches to using and
developing drugs.
Depending on your genetic makeup, some drugs may work more or less effectively for
you than they do in other people. Likewise, some drugs may produce more or fewer
side effects in you than in someone else.
It is hoped that in the near future, doctors will be able to routinely use information
about your genetic makeup to choose those drugs and drug doses that offer the
greatest chance of helping you. Pharmacogenomics may also help to save you money.
By using information about your genetic makeup, doctors soon may be able to avoid
giving you drugs that are likely to be ineffective or cause bad side effects. Most people
today are given a drug dose determined by their BMI (body mass index).
This results in some people being:
 |
- Under treated if they metabolize
the prescribed drug quickly and
it does not have enough time to
interact and kill the cancer
cells.
- Over treated if they metabolize
the drug very slowly or not at
all and are subjected to the
drug for too long a period of
time leading to toxic levels in
their bodies.
|
| Image provided by CISN archives. All rights reserved. |
|
Examples in use today:
- 5-FU metabolism - 1 in 4 individuals carries variations in either the DPYD or
TYMS genes that will increase their risk of dose-limiting toxicity. These variations
confer up to a 60% risk of toxicity to 5-FU-related therapies.
- "...given the large number of patients treated each year with 5-FU...and the
human and economical cost of grade 3 and 4 toxic side effects, pretherapeutic
detection of DPD deficiency should be considered."1 Reference: 1. Morel A, Boisdron-
Celle M, Fey L, et al. Clinical relevance of different dihydropyrimidine dehydrogenase gene single
nucleotide polymorphisms on 5-fluorouracil tolerance. Mol Cancer Ther. 2006;5:2895-2904.
- The breast cancer drug trastuzumab (Herceptin) is a therapy that works only
for women whose tumors have a particular genetic profile that leads to
overproduction of a protein called HER2.
- The U.S. Food and Drug Administration (FDA) recommends genetic testing
before giving the chemotherapy drug mercaptopurine (Purinethol) to patients
with acute lymphoblastic leukemia. Some people have a genetic variant that
interferes with their ability to process the drug. This processing problem can
cause severe side effects.
- The FDA also advises doctors to test colon cancer patients for certain
genetic variants before administering irinotecan (Camptosar). The reasoning
is that patients with one particular variant in the UGT gene may not be able to
clear the drug from their bodies as quickly as others, resulting in severe diarrhea
and increased infection risk. Such patients may need to receive lower doses of
the drug.
- Patients with variant forms of the gene CYP2D6 may not receive full benefit
from tamoxifen because it is metabolized too slowly. On Oct 18, 2006 the
Subcommittee for Clinical Pharmacology recommended relabeling tamoxifen to
include information about this gene in the package insert.
- Recent studies suggest that taking antidepressants such as Paxil, Prozac,
etc., can decrease the effectiveness of tamoxifen, because these drugs
compete for the CYP2D6 enzyme. This enzyme is needed to metabolize
tamoxifen into its active form endoxifen.
- Chemicals in grapefruit juice and grapefruit pulp interfere with the enzymes
that break down (metabolize) various drugs in the digestive system. The result
can be excessively high levels of these drugs in the blood and an increased risk
of potentially serious side effects. Tell your doctor if you drink/eat a lot of
grapefruit products.
Can Pharmacogenomics Be Used To Develop New Drugs?
Besides improving the ways in which existing drugs are used, genome research could
lead to the development of better drugs. The goal is to produce new drugs that are
highly effective without causing serious side effects. SNP screenings will benefit drug
development and testing because pharmaceutical companies could:
- Exclude from clinical trials those people whose pharmacogenomic screening
showed that the drug being tested would be harmful or ineffective for them.
- Excluding these people will increase the chance that a drug will show
evidence of effectiveness in a particular population thus increasing the
chance that the same drug will make it into the marketplace.
- Pre-screening clinical trial subjects could also allow the clinical trials to use
fewer subjects, proceed faster, and therefore be less expensive, resulting in
consumer benefit from reduced drug costs.
- Assess an individual's reaction to a drug before it is prescribed will increase a
physician's confidence when prescribing the drug and the patient's confidence
when taking the drug. This in turn should encourage the development of new
drugs tested in a similar manner.
- Breathe new life into some drugs that were shelved during the development
process.
What Are Some Of The Barriers To Pharmacogenomic Progress?
Pharmacogenomics is a developing research field that is still in its infancy. Several of
the following barriers will have to be overcome before many pharmacogenomics
benefits can be realized.
Further complicating the process is our limited knowledge of which genes are
involved with each drug response. Since many genes are likely to influence
responses, uncovering the big picture on the impact of gene variations is
highly time-consuming and complicated.
- Limited drug alternatives - Only one or two approved drugs may be available
for treatment of a particular condition. If patients have gene variations that
prevent them from using these drugs, they may be left without any
alternatives for treatment.
- Disincentives for drug companies to make multiple pharmacogenomic
products - Most pharmaceutical companies have been successful with their "one size fits all" approach to drug development. Since it costs hundreds of
millions of dollars to bring a drug to market, will these companies be willing to
develop alternative drugs that serve only a small portion of the population?
- Healthcare provider education - Introducing multiple pharmacogenomic
products to treat the same condition for different population subsets
undoubtedly will complicate the process of prescribing and dispensing drugs.
Physicians must execute an extra diagnostic step to determine which drug is
best suited to each patient. To interpret the diagnostic accurately and
recommend the best course of treatment for each patient, all prescribing
physicians, regardless of specialty, will need a better understanding of
genetics.