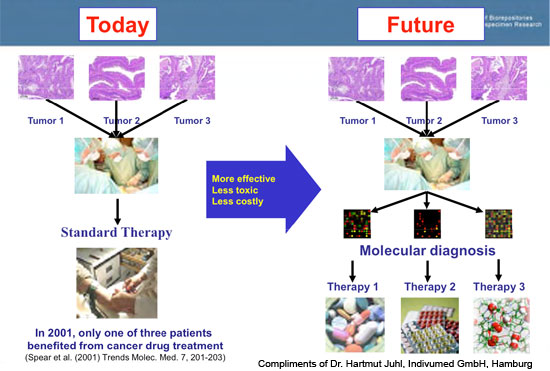
To fully implement this vision, translational research requires researchers and clinicians to have ready access to two critical types of information:
- Clinical information, including data in medical records, pathology reports and diagnostic labs, clinical trials systems and study participant questionnaires.
- Biomolecular information, including genomics, proteomics, medical imaging and other molecular and cellular research data.
New belief systems and structures are needed
Collaboration between researchers, data sharing, data integration and standards are
integral to translational research. Only by seamlessly structuring and integrating
these data types into one whole will the complex and underlying causes and
outcomes of disease be revealed, and effective prevention, early detection and
personalized treatments be realized.
This is a departure from the process of basic research in which each researcher usually works independently and only shares discoveries through publication of results. To
take an idea from concept through publication takes many years during which only a
few people have access to this work.
Translational research demands that
researchers and clinicians work in a collaborative, multidisciplinary approach, sharing as they
discover. This newer approach of more collaboration is now being utilized in all stages of the research process.
The 'NIH Roadmap' is a project that is attempting to assist in these efforts.
The image is from a recommendation report to the NCI from the NCI Translational Research Working
Group. It illustrates the steps involved along the translational pathway.
For more information on the
NCI roadmap:
http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp