Correlative Science - page 2
Clinical Applications of Correlative Science - cont.
Leadership is needed in the following areas
- Determining whether correlative marker collection should be mandatory for
patients to enter a clinical trial.
- The future of targeted therapy depends on tying tissue biomarker research to patient outcomes. The best way to do this is within a clinical trial where outcomes are tracked and measured.
- Is this 'future good' explanation enough reason to require 'current' trial participants mandatory tissue donation?
- Creation of standardization guidelines needed for patient consent, collection
and processing of samples.
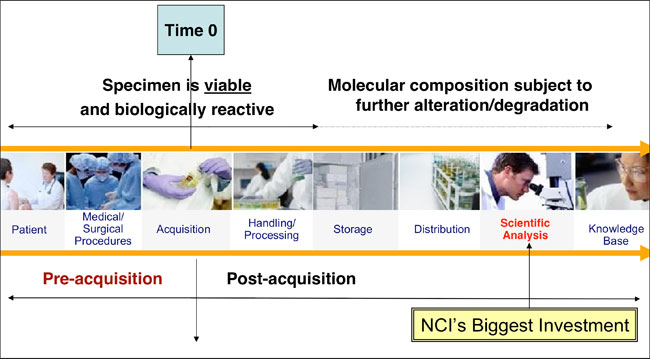
- See image below showing various steps that tissue moves through. If all of these are not standardized, you may end up comparing apples to oranges as values may change due to poor processing and storage.
- Standardizing clinical information from patients donating samples so that
information can be tied to outcomes. Privacy issues are involved as well and
must be addressed.
- Again if clinical information is not identical across samples you may end up comparing apples to oranges with no transferable new knowledge gained.
- Developing better guidelines: some FDA guidelines do not adequately
distinguish biomarkers used as surrogate endpoints from those used for
treatment selection and are inappropriate for the latter applications.
- Treatment selection using biomarkers is based on responders vs. non-responders and/or different side effect profiles.
- Surrogate endpoints are entirely different and have a focus on time to recurrence and/or survival.
- Determining whether groups that receive government funding should be able to keep the data derived from correlative science studies private rather than adding it to the caBIG data bank; examples of these groups are cooperative groups, cancer centers and individual academic researchers.
- Creating a mechanism so that the data used to develop a predictive classifier must be distinct from the data used to test hypotheses about treatment effect in subsets determined by the classifier.