|
|
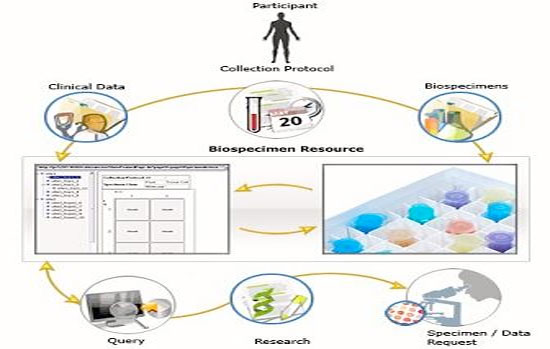
Biobanks must carefully store and document access to
samples and donor information. The samples must be
maintained reliably with minimal deterioration over time,
and they must be protected from physical damage, both
accidental and intentional.
The registration of each sample entering and exiting the
system is centrally stored, usually on a computer-based
system that can be backed up frequently. The physical
location of each sample is noted to allow the rapid location
of specimens.
Archival systems de-identify samples to respect the privacy
of donors and allow blinding of researchers during analysis. |
The database, including clinical data, is kept separately with a secure method to link
clinical information to tissue samples.
Room temperature storage of samples is sometimes used, and was developed in
response to perceived disadvantages of low-temperature storage, such as high costs
and potential for freezer failure. Current systems are small and are capable of storing
nearly 40,000 samples in about one tenth of the space required by a -80 °C
(-112 °F) freezer. Replicates or split samples are often stored in separate locations
for security.
In the news:
Biobanks are listed as number 8 in the "10 Ideas Changing the World Right Now"
in the TIME magazine issue that hit the news stands March 23, 2009.
"Now that major banks in the U.S. are getting by on a government bailout, the idea of
creating yet another repository to safeguard your most valuable assets might seem
downright ludicrous. And even irresponsible.
 |
|
But that's exactly what some federal officials
are hoping to do.
Relax - it's not your money they're after. It's
your blood.
Folks at the National Cancer Institute (NCI)
are heading up an effort to establish the
U.S.'s first national biobank - a safe house
for tissue samples, tumor cells, DNA and,
yes, even blood - that would be used for
research into new treatments for diseases. |
Think of it as an organic bank account. You put your biomaterial in and earn medical
interest in the form of knowledge and therapies that grow out of that deposit - no
monetary reward, just the potential that you might benefit from the accumulated
data at some later date. (Sorry, no shiny new toaster to inspire you to open up such
an account either - just an appeal to the greater medical good.)"
- Quote and image from the Time.com article,
10 Ideas Changing the World Right Now
To read the entire article: Click Here
Examples of groups working in the Biobank field:
1) The NCI's Office of Biorepositories and Biospecimen Research (OBBR's) director,
Carolyn Compton, M.D., Ph.D., and her staff are currently developing the concept for
a new national biobank: a unique, non-profit public resource that will ensure the
adequate and continuous supply of human biospecimens and associated measurable,
high-quality data, all acquired with the highest ethical standards.
For more information: http://plan.cancer.gov/Biobanking.htm
2) The caBIGŪTissue/Biospecimen Banking and Technology Tools Knowledge Center
is an NCI-supported entity led by the Siteman Cancer Center, Washington University
at St. Louis, MO.
The Knowledge Center provides a centralized, authoritative repository of knowledge,
information, and web-based support to facilitate the ongoing development of caBIGŪ
tools, standards, and infrastructure in the tissue/biospecimen management domain.
For more information: https://cabig-kc.nci.nih.gov/Biospecimen/KC/index.php/Main_Page
3) Kaiser Permanente has launched a drive to obtain 500,000 volunteers to donate
blood and saliva for genetic analysis to study how life style, environmental factors
and genes interact to contribute to a range of diseases including cancer.
4) UK Biobank is recruiting up to half a million participants between the ages of 45
and 69 years to contribute blood samples, life-style details and medical histories for
epidemiology studies.
5) Biobank Japan is creating a DNA repository of 300,000 individuals.
Data Management
One of the first steps in ensuring compliance with international best practices
requires the adoption of a biobank data management system capable of integrating
all the heterogeneous data types associated with a sample in a secure yet accessible
manner.
Coupled with regulation and administrative requirements is the pressing need to
provide a data management platform that readily allows the selection of appropriate
patient/sample populations for studies based on heterogeneous search criteria
across epidemiological, clinical and omic data domains. Public, academic and private
data collection platforms exist today.
Ideally, the process of collecting biological specimens should be linked to a database
containing clinical information and a tracking system for stored samples. This
enables researchers to recover and be aware of the potential development of
translational research applications as well as to recover samples needed to develop
their projects very rapidly. In this context, it is particularly important to identify
samples from patients entering in clinical trials using innovative therapeutic
approaches and connect such information with biological and clinical databases.
Distribution of Samples
 |
|
Who has access to a biospecimen? What requirements
need to be met in order to receive them?
These and other questions must be part of a standard set
of requirements to ensure patient rights as well as move
the science forward as quickly as possible. |
An understanding of the balance that needs to be struck
between the interests of society and the rights of
individual patients, central to the ethical use of human
tissues and the use of clinical outcome information for research, must form the basis
for the distribution of any national tissue resource.
CISN Summary:
We at CISN support the idea that ALL biospecimen data collected with public funds
should reside in the public domain in order to best ensure the prompt development
from specimen collection to new discoveries that benefit patients.
- Standardization of collection and processing is crucial for accuracy.
- Security, privacy and confidentiality are all issues to be dealt with.