Ethical Issues
While most clinical trials are conducted in an ethical manner, a number of egregious abuses have occurred (e.g. the Tuskegee study of untreated syphilis in African American men - http://cdc.gov/tuskegee/timeline.htm) which has led to international agreement on the principles of ethical conduct of clinical trials.
The chart below summarizes these ethical principles. While these principles seem to be quite clear and simple to follow, in practice they are often difficult to implement.
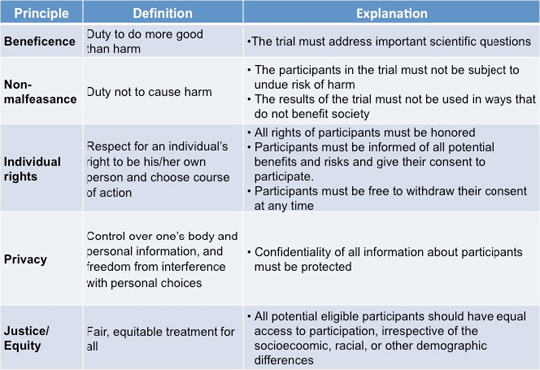 |
Ethical Principle Implementation
In order to satisfy the principle of beneficence, how important does the scientific question have to be and how likely does the possibility of positive results have to be to justify conducting a clinical trial? The answer to this question is not as straightforward as it would seem.
One-way to answer this is to ensure that at the beginning of a trial, those who are enrolling patients preserve equipoise between the experimental and control treatments. That is, to the best of their knowledge, the two treatments would be equally beneficial, and thus the trial will be conducted to determine which treatment would be best to recommend in the future.
In order to meet the principle of justice extra efforts must be made to include economically disadvantaged populations, individuals from rural areas, and (racial or ethnic) minorities since they are often under-represented in clinical trials. Not only does this violate the principle of justice, it also makes it difficult to generalize the results of such trials to populations as a whole.





