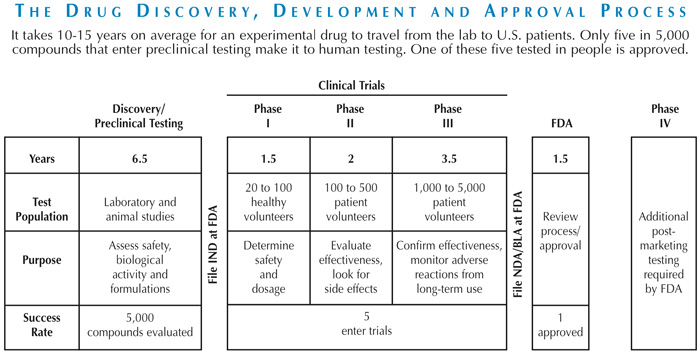
Drug development is a long, complicated and expensive endeavor. Each of the stakeholders has different and even sometimes-competing goals, so it is critically important that all aspects work as smoothly as possible.
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
The major challenges of cancer drug development:
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Copyright © 2006-2017 CISN - All Rights Reserved. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Site Design by: Cara M. Caloroso | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||