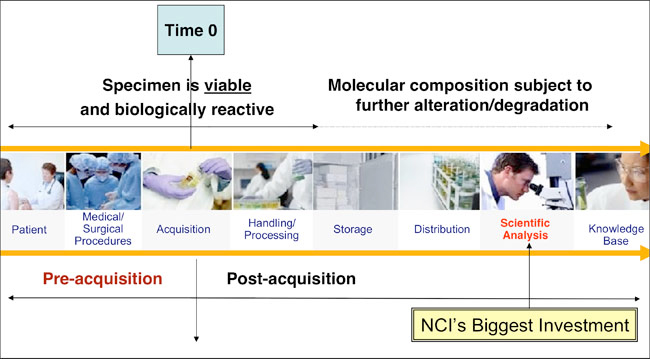
This term refers to how tissue is obtained from an individual. At the collection stage,
there are multiple factors that can affect the biomarkers present in your tissue. Called
pre-analytes, these factors may affect the tissue before it is processed, stored, or
analyzed.
Some examples of pre-analytic factors include the use of anesthesia during surgery.
The compounds used for anesthesia can infiltrate the blood supply and tumor tissue
and affect its composition.
Clamping of the veins and arteries during surgery represents another pre-analytic
factor. Clamping to reduce bleeding deprives the tumor of its blood supply, thus
altering the quality and attributes of the tissue, and has the potential to affect the
biomarkers that will be measured in the tissue. The presence of specific biomarkers
and the ability to measure such markers effectively is important because it may
suggest whether specific treatments are right for a particular patient.
Successful tissue retrieval requires the surgeon’s hand-off to knowledgeable
personnel who can handle the tissue appropriately. A trained responsible individual
must transport the tissue from the operating room to the lab where it will be
preserved.
Processing
Once the tissue is collected from the body, the next step is called processing.
| |
|
|
|
 |
|
This refers to the handling of the tissue to prepare it for testing
or storage. |
|
Image provided by CISN archives.
All rights reserved. |
| |
Laboratory technicians must be available in a timely manner to preserve the tissue or
otherwise handle it for clinical or research purposes. Temperature, time, and the
fixative used all play a crucial role in proper processing.
For example, it is important to consider the temperature of the lab in which the tissue
is held while awaiting preservation; the temperature of the storage facility; the effects
of freezing and thawing, including multiple instances of freezing and thawing the
same sample; the amount of time spent in fixative; and the time spent in storage,
since long-term storage can lead to degradation of the sample.
The optimal conditions for tissue collection, processing, and storage will depend on
the tissue type, the assays that need to be performed, and whether the tissue will be
used for a clinical (diagnostics and treatment) or a research application.
Tissue is often stored in large facilities called biobanks that may be located within
hospitals, research centers, or private facilities.
Some units may also be linked to data sources that provide information about the
variables to be studied (type of disease, outcome, treatment received, individual
characteristics).
In its “Best Practices for Biospecimen Storage,” the National Cancer Institute calls for:
- standardized protocols for storage, depending on the tissue type extracted
(e.g., wet tissue, frozen tissue, paraffin-embedded tissue, blood, serum, urine)
and the biomolecules to be analyzed (e.g., RNA, DNA, proteins, lipids).
- security warnings that will monitor the function of the storage equipment and
alert personnel to any power failures limiting biobank access to specific individuals who are knowledgeable about
privacy procedures.
Tissue testing or analysis also needs to be standardized to enable accurate
comparison of results.
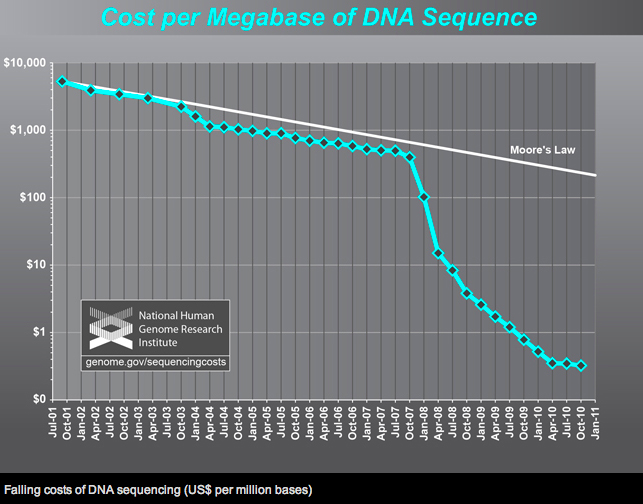
Advances in genetic sequencing and molecular analytic technologies have increased
the call for high-quality biospecimens. Some analyses can be performed with minute
amounts of tissue. Tests that previously could only be performed with fresh tissue can
now be conducted with paraffin-embedded tissue.