Although our genes influence our risk for cancer, much of the difference in
cancer risk is due to factors that are not inherited. Although there is little you
can do if you do have a specific genetic mutation, there are still steps that you
can take to reduce your risk of developing cancer.
Such measures include avoiding tobacco products, keeping a healthy weight,
staying active throughout life, and eating a healthful diet, all of which may
reduce a person’s lifetime risk of developing cancer. These same behaviors are
also associated with a lower risk of developing heart disease and diabetes.
Although we all have the ability to make healthy choices, the social, physical, and
economic environments in which we live may positively or negatively impact our
ability to “stick with” our well-intended plans. Nevertheless, is it important to
realize that you are in control.4
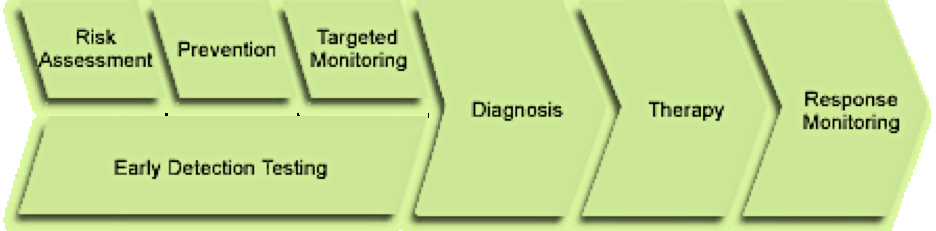
Targeted monitoring
Personalized medicine introduces the ability to use markers that may signal risk
or detect disease before symptoms appear. Women with certain BRCA gene
variations have an increased risk of developing breast cancer.
Increasing the frequency of mammograms may be recommended to help early
detection, or preventive surgery or chemoprevention may be considered for
possible risk reduction. Genetic markers are also currently being used to
facilitate safer and more effective drug dosing and scheduling.3
Diagnosis
Fulfilling the promise of personalized medicine requires that these findings be
translated into precise diagnostic tests and targeted therapies.
In current practice, cancer diagnosis is primarily based on symptoms (clinical
presentation) and by your pathology report. New molecular tools may help to
characterize cancer subtypes that cannot currently be distinguished clinically and may
provide predictive information concerning disease outcome, aiding in guiding
treatment decisions.
Below are types of testing now being used to provide your doctors with the information
they need to make an accurate diagnosis of your cancer type and stage.
Hereditary Gene Testing
Personalized medicine is being used today in the testing of inherited genetic
mutations. For example, specific BRCA1 and BRCA2 gene mutations are
implicated in familial breast and ovarian cancer. Genetic testing can be provided
to determine individuals at increased cancer risk due to such BRCA mutations,
which may prompt more intense monitoring and consideration of prophylactic
therapy.
Somatic Gene Testing
Personalized medicine is now being used to detect “somatic” or non-inherited
mutations in cancer. Somatic mutations refer to acquired gene mutations or
DNA changes that occur after conception. Some examples are the following:
- Mutations of the KRAS gene in advanced colorectal cancer are predictive of
a poor response to therapy with anti-EGFR (anti-epidermal growth factor
antibody) agents, an important finding that now guides treatment
decisions for patients with and without KRAS mutations
- The discovery of a chromosomal translocation in chronic myelogenous
leukemia (CML), led to the development of a new drug called Gleevac that
targets the enzyme produced as a result of that mutation.
Diagnostic testing – a few examples
- Oncotype DX is a diagnostic test to detect patterns of genetic
abnormalities within tumors. These patterns predict the probable rate of
recurrence and can help guide treatment decisions. This test is available
for both breast and colon cancers.
For more information on this click here.
- Testing for the overexpression of Her2 gives physicians information to make
treatment recommendations. For more information on this click here.
- Testing for estrogen and progesterone status also helps determine
treatment recommendations. For more information on this click here.
Therapy
New molecular tools may also provide doctors with information on which drugs their
patients may or may not respond to and/or the likelihood of their developing adverse
effects associated with certain agents.
Targeted Therapy
Targeted therapy is the use of agents designed to target specific mutated molecular
pathways in a subset of patients with a given cancer type.
- As noted earlier, trastuzumab (Herceptin®) and additional anti-HER2
agents may be used to treat patients with HER-2-neu positive breast
cancer and cancers of the stomach and gastroesophageal junction.
- Also mentioned earlier, imatinib (Gleevec®) is used to treat chronic
myeloid leukemia that has the Philadelphia chromosome.
- Using a whole genome screen to identify genes active in cutaneous T-cell
lymphoma (CTCL), scientists found that presence of the protein HR23B
predicted response to the drug vorinostat (Zolinza®).
- 5-fluorouracil (5-FU) is a commonly used chemotherapy agent.
Dihydropyrimidine dehydrogenase (DPD) is the primary enzyme that determines the
breakdown of 5-FU. Some people have genetic variations, causing the DPD enzyme to
be less active or inactive, limiting the metabolism of 5-FU.
As a result, people with such DPD mutations have a higher risk of developing severe or
even fatal reactions to 5-FU. Screening for DPD mutation and direct measurement of
DPD activity prior to treatment with 5-FU will enable proper adjustment of dosage to
prevent a dangerous adverse reaction.
Response Monitoring
To truly wipe out cancer cells within the body, it is not enough to have effective drugs
that target some of the cancer growth pathways. It is important to have a way of
monitoring the cancer itself, so that drug therapy can be adjusted should the tumor
change or evolve.
Response monitoring may be done by measuring tumor markers (biomarkers).
“Content Developed September 1, 2012” |