Tumor markers can be classified into two groups:
- Cancer-specific markers are related to a particular type of cancer. The
tumor itself produces the marker. Cancer-specific markers can be useful
in the follow-up of treated patients to track progress of the disease or
response to treatment. Examples include:
a. CEA (which may be used to monitor patients treated for colorectal
cancer)
b. CA19-9 (initially developed used in colon cancer, but found to be
very sensitive for pancreatic cancer)
c. CA-125 (a marker that may be used to monitor women with the
most common type of ovarian cancer during and after active
treatment).
- Tissue-specific markers are related to specific tissue. Elevated levels may
suggest abnormalities of specific tissue, such as the presence of cancer. An
example is:
a. PSA, which may be elevated in the blood when there is noncancerous
prostate growth or with prostate cancer. In addition, PSA
also tends to be elevated in older men and in cases where there is
inflammation or infection of the prostate.
Tumor markers are not always reliable for the following reasons:
- Normal cells as well as cancer cells can make most tumor markers.
- Tumor markers may also be associated with noncancerous conditions.
- Tumor markers are not always present in early-stage disease.
- People with cancer may never have elevated tumor markers.
- Even when tumor marker levels are high, cancer may not be present.
- Tumor markers that may be helpful in monitoring some cancer patients
during and after treatment most often are not reliable in detecting cancer.
Because abnormal tumor marker levels may only suggest the presence of cancer,
other scientific tests are usually required before confirming a cancer diagnosis.
Genetics
Genetics is the science of genes and the transmission and variation of inherited traits—
in other words, the process by which parents may pass certain genetic traits to their
children. Characteristics determined by genes range from eye color, hair color, and
height to inheritance of or predisposition for specific diseases.
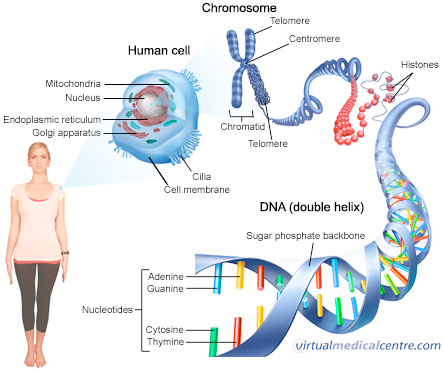
”Genome” refers to an organism’s complete set of DNA, including all of its genes. In
humans, a copy of the entire genome - which is more than 3 billion DNA base pairs – is
contained in every cell that has a nucleus. Genomics
Genomics is the study of a person’s complete DNA sequence - including genes and
“noncoding” DNA segments in the chromosomes and how those genes interact with
each other, as well as the internal and external environments they are exposed to.
This includes the study of gene mutations, both those that are passed from parents to
children (inherited) and those that happen during your lifetime (somatic). It is these
mutations that contribute to the development and spread of cancer.
Pharmacogenetics
Pharmacogenetics is a specialty field within the study of genomics. It is generally
regarded as the study of genetic variation that can affect an individual’s responses to
certain drugs. Pharmacogenetics uses information about a person's genetic makeup, or
genome, to help determine the drugs that are likely to work best for that particular
person and/or that may be likely to lead to certain adverse effects.
For certain drugs, pharmacogenetics may help to determine whether patients are rapid
or slow metabolizers (referring to how long the drug stays in your body) as well as
whether they are responders or non-responders (meaning whether the drug works
for you).
Having the ability to predict a person’s individual response to a drug, both in terms of
therapy benefit and the likelihood for adverse events, will play a crucial role in:
- Preventing the use of a drug that is likely to cause serious effects for a particular
patient without benefit
- Guiding the selection of optimal therapies that are most likely to benefit a
specific patient
Deaths from drug side effects are one of the top ten leading causes of death, so this
field is very important.
Epigenetics
For decades, scientists and doctors assumed that cancer was caused by irreversible
damage to some critical stretch of DNA within one's genome. But in the last few years,
a much more complex picture has emerged, one that shows that some cancers are
caused by epigenetic changes-tiny chemical tags that accumulate over time and can
turn genes on or off rather than mutate them.
The word "epigenetic" literally means "in addition to changes in genetic sequence." It is
used to refer to any process that alters gene activity without changing mutating the
DNA sequence. Experiments show that epigenetic changes, unlike mutations, can be
reversed.
Proteomics
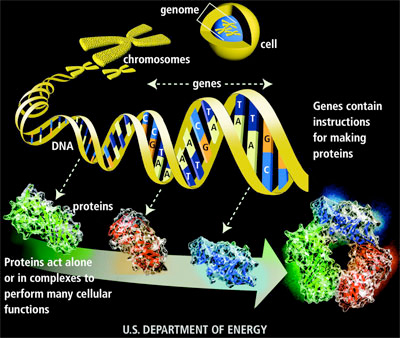
Proteomics is the study of the structure, functions, and interactions of proteins. While
genes are the “recipe” of the cell, containing all the instructions for assembly, proteins
are the products of these recipes, functioning as the cellular engines that drive both
normal and disease physiology.
| |
|
|
 |
|
Proteomics is much more complicated than
genomics. While an organism’s genome is
more or less constant, the proteome - or set
of proteins expressed by the genome -
differs from cell to cell and from minute to
minute, depending on the activity of specific
genes.
This makes interpreting protein
measurements very difficult. |
| Image Courtesy of U.S. Human Genome Project |
A breakthrough in cancer treatment was the discovery that tumors “leak” proteins and
other molecules into blood, urine, and other accessible body fluids. The greatest
promise for the early detection and treatment of cancer is to collect such fluids from
patients and test them for the presence of cancer-related molecules, called cancer
biomarkers or tumor markers.
- The vast number of proteins that exist in the body can make it difficult to
identify and describe them.
- Proteins are continually moving and undergoing changes.
- Proteins exist in a wide range of concentration in the body.
- Lack of standardization – Laboratories across the county collect, store, and
study proteins in different ways. This lack of standardization makes it difficult
to accurately compare results from one laboratory to another.
Metabolomics
Metabolomics is the systematic study of the unique chemical fingerprints that cells
leave behind. More specifically, it is the study of how your body responds to drugs,
environmental changes and diseases. Metabolomics is an extension of genomics (study
of genes) and proteomics (study of proteins).
If metabolomic information could be translated into better diagnostic tests, it might
have the potential to impact clinical practice, and it might lead to more targeted
biomarkers that improves care.
| |
|
|
|
 |
|
If you move from the top of the image to the
bottom you get more precise information on
what is happening in your body. So
metabolites are a closer marker to how cells
are functioning than genes or proteins.
Therefore, measuring them may be more helpful
in improving disease detection, treatment and
monitoring than measuring either genes or
proteins. |
|
| Image courtesy of Royston Goodacre School of Chemistry, The University of Manchester |
«Click Here to go back to main content. |